Current Research Projects
Metabolic behaviors of intracellular S. Typhimurium
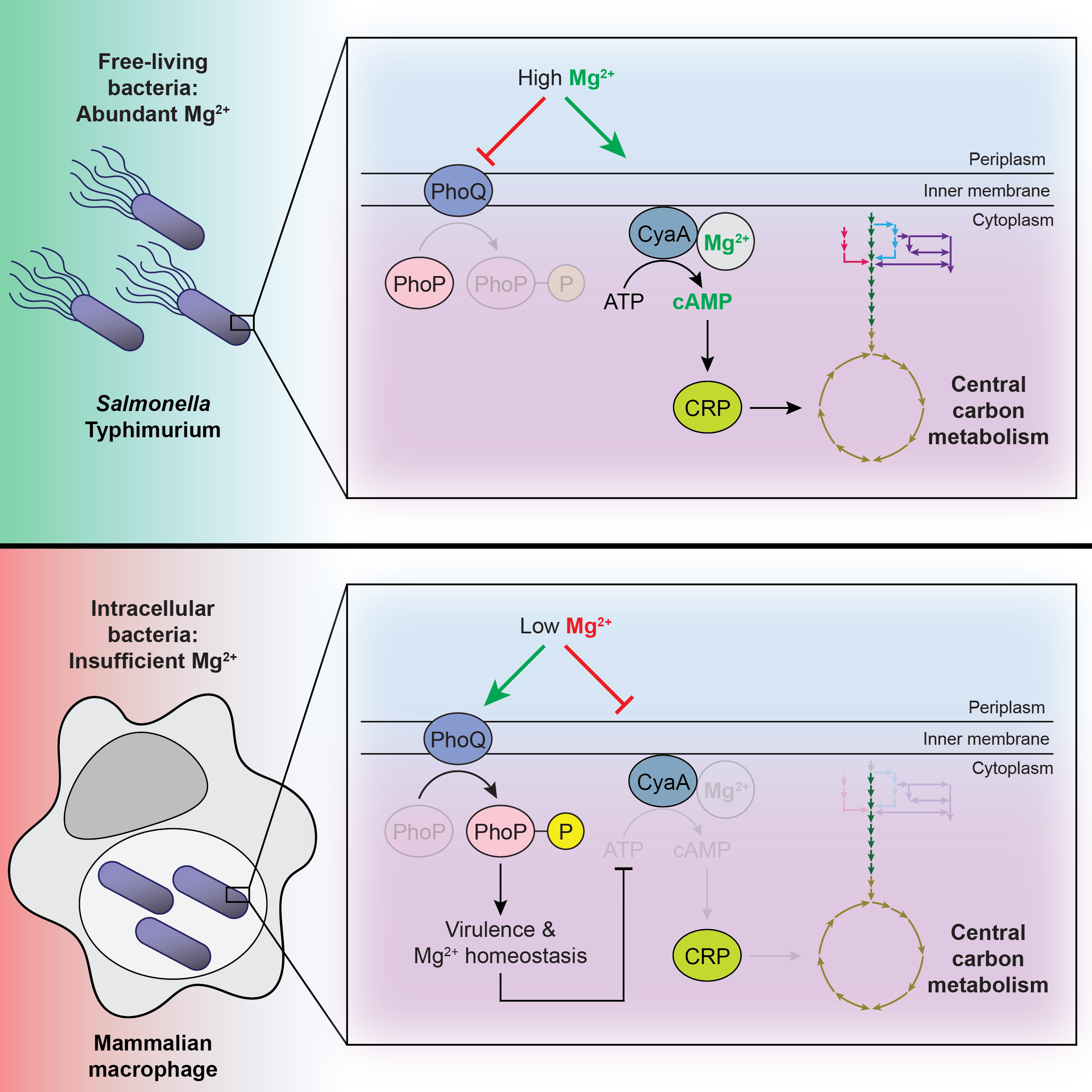
Metabolism is the foundation of cell physiology because all biological molecules are synthesized or transformed by metabolic processes. During systemic infection, S. Typhimurium resides within splenic macrophages. Inside macrophages, S. Typhimurium faces starvation for magnesium (Mg2+), otherwise the most abundant divalent cation in all cells. Mg2+ starvation slows growth and activates virulence traits which promote pathogen survival. We revealed that Mg2+ starvation dramatically alters S. Typhimurium metabolism by decreasing synthesis of the ubiquitous nucleotide second messenger, 3’,5’-cyclic adenosine monophosphate (cAMP), allosteric activator of carbohydrate utilization master regulator, the cAMP receptor protein (CRP). Decreased CRP-cAMP amounts shift S. Typhimurium’s carbon source preference inside macrophages, explaining their utilization of non-glucose carbon sources in this environment.
We are now interested in understanding various aspects of this metabolic regulation. For example, cAMP abundance is controlled at the level of synthesis, degradation, and excretion. How does Mg2+ control the additional mechanisms regulating cAMP concentration in the cell and does this impinge on CRP activity and metabolism? CRP-cAMP dictates carbon source utilization by activating transcription of genes encoding various carbohydrate importers, yet many post-transcriptional mechanisms control importer abundance and activity. Does Mg2+ control post-transcriptional regulation of S. Typhimurium’s carbon source preference? Despite low CRP-cAMP amounts during Mg2+ starvation, some CRP-cAMP targets are transcriptionally activated rather than repressed. How does CRP-cAMP exert transcriptional control over some genes but not others when cAMP abundance is low?
The metabolic basis of antibiotic tolerance
Metabolic state predicts antibiotic susceptibility and inside macrophages S. Typhimurium exhibits increased tolerance to clinically relevant antibiotics due to slow growth. We hypothesize that when S. Typhimurium faces conditions that slow growth, such as Mg2+ starvation, accompanying metabolic changes provoke antibiotic tolerance. We will explore the antibiotic susceptibility of S. Typhimurium under infection-relevant conditions and reveal how the molecular mechanisms governing metabolism control antibiotic susceptibility.
Control of cell envelope homeostasis
Inside macrophages, S. Typhimurium changes various aspects of the cell envelope to resist antibacterial mechanisms of the host cell. This includes modifications to two major cell envelope constituents in Gram-negative bacteria: lipopolysaccharide and peptidoglycan. Chemical modification of these critical cell envelope structures are well understood at the molecular and biochemical level. However, it is assumed that the metabolic substrates for these modifications are never limiting. We hypothesize that metabolic alterations favor these modifications under infection-relevant conditions. We will elucidate the metabolic foundation for virulence-associated changes to the cell envelope that promote S. Typhimurium survival.